Article published in 2nd edition of Canal Solar Magazine
Electric batteries are electrochemical energy stores. This means that in these devices energy is stored or discharged through chemical reactions.
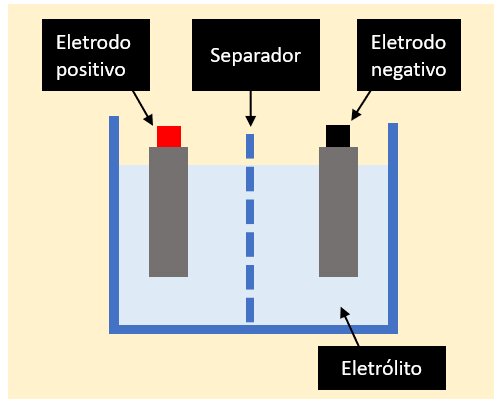
Batteries always have a very similar structure, being composed of cells made up of two electrodes, an electrolytic substance (also called electrolyte, usually a liquid solution) and a separator, as shown in Figure 1.
What differentiates the different battery technologies are the materials used in the electrodes, the electrolyte substances and the construction aspects.
The most common batteries are lead-acid (Pb), lead-carbon, lithium-ion (Li-ion), nickel-cadmium (NiCd), nickel-metal hydride (NiMH), nickel-sodium chloride (NaNiCl2), sodium and sulfur (NaS) and flow batteries (which may employ different chemical elements).
The subject of electrochemical batteries is quite rich and extensive.
The objective of this article is to carry out a brief review of electrochemical batteries, with an emphasis on the technologies currently most used or most promising for use in photovoltaic systems and electrical energy storage systems in general.
Among the technologies most used in energy systems, lead-acid and lithium-ion batteries stand out. Lead-acid batteries are an old technology that is still widely used, especially in off-grid energy systems.
Lithium-ion batteries are currently the big stars of the storage market, with many applications in energy systems and electric mobility.
The need for more efficient, more durable, more compact, lighter and cheaper batteries has led to the incessant search for new materials and new electrochemical storage technologies.
Recently, the growth of interest in electric mobility has strongly boosted research in the area of solid electrolytes, which provide lithium batteries with greater durability, greater energy density and greater safety.
In the group of alternative technologies, flow batteries and liquid salt batteries stand out, which are still little known, but very promising for large-scale storage applications.
Battery characteristics
Batteries from different technologies can have very different characteristics. Batteries can have high storage capacity and high energy density, but their life cycle can be short.
On the other hand, they can be developed to be highly durable, but they can be heavy and bulky. They can also have high capacity and long durability, but their cost can be prohibitive for most applications.
Batteries can be evaluated according to specific energy (Wh/kg), energy density (Wh/L), charge capacity (Ah), acceptable depth of discharge (DOD – depth of discharge), the lifetime (related to the number of charge and discharge cycles that the battery supports), the ability to withstand high temperatures (which affect the lifetime and safety of operation), the specific power (W/kg) and the rate C-rate, which determines the speed at which batteries can be charged or discharged.
There are determining characteristics depending on the type of application. For stationary applications in renewable energy and electrical systems, the main characteristics are generally cost, number of work cycles, efficiency and robustness – the latter associated with the ability to withstand high temperatures, overloads or deep discharges.
For mobile applications, specific energy (mass), energy density (volume) and charge and discharge speed are important characteristics.
There is no ideal battery, but there is the most suitable battery for each application, taking into account technical, economic, logistical and even social aspects.
For example, lead acid batteries are very suitable for off-grid photovoltaic systems used to serve less privileged populations in remote and difficult to access locations, almost always in hot regions of the planet.
Lead acid batteries withstand high operating temperatures (up to 60 OC), they are cheap, withstand occasional deep discharges, are easy to recycle and robust, not requiring specialized maintenance or complex electronic monitoring and control systems. The same cannot be said about lithium batteries, which are highly sought after for modern storage systems and electric vehicles, but are very complicated to install in places where technical assistance is difficult or often unfeasible.
Figure 2 illustrates the relationship between specific energy (Wh/kg) and energy density (Wh/L) of some common battery technologies. The disadvantage is observed with respect to the mass and volume of lead acid batteries compared to lithium batteries. This disadvantage, however, does not take into account other factors that can make lead acid batteries more attractive, as stated in the previous paragraph. On the other hand, if we are looking for more compact and lighter systems, lithium technology is unmatched – which is why it currently dominates the energy storage market.
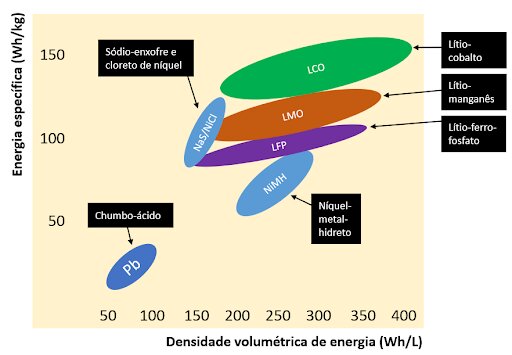
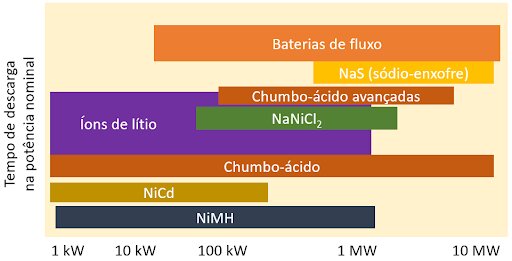
Figure 3 illustrates the relationship between charge/discharge time and the power of the systems in which these batteries can be used, including mature and widely used technologies (such as lead acid and lithium ion) and technologies still under development or little known (such as flow batteries).
Interpreting Figure 3, we realize that flow and liquid salt batteries are only viable in large storage systems, in which their technical complexity and high response time are not limiting factors.
Lithium-ion batteries have moderate response speed and occupy an important position, suitable for small and large storage systems.
Lead acid batteries, due to their reduced cost, their robustness and their satisfactory response speed, are still preferred in many small power systems, and can also reach large-scale applications, although they are preferably replaced by lithium batteries or others with ability to perform a greater number of operation cycles.
Lead acid batteries
Lead acid batteries are the oldest rechargeable batteries found on the market. They emerged at the beginning of the last century, in the 1900s, and to this day they remain preferred in many applications due to their robustness and low cost.
Their main disadvantages are their low energy density (they are heavy and bulky) and their short lifespan, not accepting a large number of charge and discharge cycles.
They also have the disadvantage of low discharge depth, which is typically limited to 80% in extreme cases or 20% in regular operation, for greater longevity. Excessive discharge degrades the battery's electrodes, which reduces its ability to store energy and limits its useful life.
Lead-acid batteries require constant maintenance of their state of charge and must always be stored at their maximum state of charge using the floating technique (maintaining the charge with a small electrical current, sufficient to cancel the effect of self-discharge).
These batteries can be found in different versions. The most common are ventilated batteries, which use liquid electrolyte, valve-regulated gel batteries (VRLA) and batteries with electrolyte embedded in a fiberglass blanket (known as AGM – absorbent glass mat), which have intermediate performance and reduced cost compared to gel batteries.
Valve-regulated batteries are practically sealed, which prevents electrolyte leakage and drying out. The valve acts to release gases in overload situations.
Some lead acid batteries are designed for stationary industrial applications and can accept deeper discharge cycles. There is also a more modern version, which is the lead-carbon battery. Carbon-based materials added to the electrodes provide higher charge and discharge currents, greater energy density and extended service life.
An advantage of lead-acid batteries (in any of their variations) is that they do not require a sophisticated charge management system (as is the case with lithium batteries, which we will see below). Lead batteries are much less likely to catch fire and explode when overcharged because their electrolyte is not flammable like that of lithium batteries.
Furthermore, slight overcharging is not dangerous in this type of battery. Some charge controllers even have an equalization function that slightly overcharges the battery or battery bank, causing all cells to reach full charge.
During the equalization process, the cells that eventually become fully charged before the others will have their voltage slightly increased, without risk, while the current flows normally through the series association of elements. In this way, we can say that lead batteries have the ability to equalize naturally and small imbalances between the cells of a battery or between the batteries in a bank do not pose a risk.
Figure 4 illustrates modern versions of lead-acid batteries, with the addition of carbon to the electrodes, among other features, that make them more durable and more suitable for application in stationary energy systems.
The sealed battery, shown on the left (in Figure 4), has the advantage of not requiring maintenance and has a useful life of over 1,000 cycles under normal temperature conditions and when operated with a discharge depth of around 20% (recommended limit for this type battery).
The ventilated version, shown on the right, supports more than 6,000 cycles of operation under the same conditions, with the need for periodic replacement of the electrolyte level.

Lithium-Ion Batteries
Currently, the most commercially successful batteries are lithium-ion batteries. After being adopted in portable electronic equipment, lithium-ion technology has reached industrial applications, electrical power systems and electric vehicles.
Lithium batteries outperform many other types of rechargeable batteries in several aspects such as energy storage capacity, number of operating cycles, charging speed and cost-benefit. Currently its only problem is safety, which is compromised by the flammable electrolyte, which can catch fire at high temperatures, which requires the use of electronic control and monitoring systems.
Lithium is the lightest of all metals and has the highest electrochemical potential, providing greater energy density per volume and mass compared to other known battery technologies, as shown in Figure 2.
Lithium-ion technology has enabled the advancement of the use of energy storage systems, mainly associated with intermittent renewable sources (solar and wind), and is also responsible for the popularization of electric vehicles.
Lithium-ion batteries applied to electrical power systems and electric vehicles are liquid types. These batteries employ the traditional construction of an electrochemical battery, with two electrodes immersed in a liquid electrolyte solution, as shown in Figure 1.
A separator (porous insulating material) is used to mechanically distance the electrodes, while allowing the free circulation of ions through the liquid electrolyte.
The main characteristic of the electrolyte solution is the ability to allow the conduction of ionic current (formed by ions, which are atoms with an excess or lack of electrons), while not allowing the passage of electrons (as occurs in conductive materials). The exchange of ions between the positive and negative electrodes is the basis of how electrochemical batteries work.
Research into lithium batteries dates back to the 1970s and the technology became mature and began to be used commercially around the 1990s.
Lithium polymer batteries (with polymer electrolyte) today equip cell phones, computers and all types of mobile devices, having replaced the old nickel-cadmium batteries, which had as their main problem the “memory effect”, which gradually reduced the storage capacity. when batteries were recharged before they were completely discharged.
Compared to their older nickel-cadmium and, especially, lead-acid rivals, lithium-ion batteries have higher energy density (store more energy per volume), have a low self-discharge coefficient and support a greater number of batteries. charge and discharge cycles, which translates into a prolonged useful life.
Around the beginning of the 2000s, lithium batteries began to be used in the automotive industry. Around the year 2010, lithium-ion batteries gained interest in electrical energy storage, both in residential applications and in large ESS systems (energy storage systems), largely due to the increase, on a global scale, in the use of intermittent renewable sources (solar and wind).
Lithium-ion batteries can have different performance, lifespan and cost depending on how they are manufactured. Several materials have been proposed, mainly with regard to electrodes.
Typically, a lithium battery is composed of a lithium metal-based electrode forming the positive terminal of the battery and a carbon (graphite) electrode forming the negative terminal.
The lithium-based electrode can come in different configurations, depending on the technology used. The most commonly used materials in the manufacture of lithium cells and the main characteristics of these batteries are listed below:
- Lithium and cobalt oxide (LCO): High specific energy (Wh/kg), with good storage capacity and satisfactory lifetime (number of cycles) for applications in electronic equipment, with the disadvantage of low specific power (W/kg), which reduces charging speeds and discharge;
- Lithium and manganese oxide (LMO): Allows high charging and discharging currents, with low specific energy (Wh/kg), which translates into reduced storage capacity;
- Lithium, nickel, manganese and cobalt (NMC): Combines characteristics of LCO and LMO batteries. Furthermore, the presence of nickel in the composition helps to increase the specific energy, providing greater storage capacity. Nickel, manganese and cobalt can be used in different proportions, depending on the type of application (to favor one or another characteristic). In general, the result of this combination is a battery with good performance, good storage capacity, good useful life and moderate cost. This type of battery has been widely used in electric vehicles and is also suitable for stationary energy storage systems.;
- Lithium, iron and phosphate (LFP): The LFP combination provides batteries with good dynamic performance (charge and discharge speed), increased service life and greater safety due to their good thermal stability. The absence of nickel and cobalt in their composition reduces the cost and increases the availability of these batteries for mass manufacturing. Although its storage capacity is not the highest, it has been adopted by manufacturers of electric vehicles and energy storage systems due to its several advantageous characteristics, especially its low cost and good robustness.;
- Lithium and titanium (LTO): The name refers to batteries that have titanium and lithium in one of the electrodes, replacing carbon, while the second electrode is the same used in one of the other types (such as NMC – lithium, manganese and cobalt). Despite the low specific energy (which translates into reduced storage capacity), this combination presents good dynamic performance, good safety and a greatly increased useful life. Batteries of this type can accept more than 10,000 cycles of operation with 100% depth of discharge, while other types of lithium batteries accept around 2,000 cycles.
Current research related to lithium-ion batteries is looking for new materials and manufacturing methods that can increase lifespan, energy density, safety and charging speed – while reducing the cost of production.
Recent developments have moved towards solid-state lithium batteries, in which the liquid electrolyte is replaced by a solid material that has the property of conducting ions.
Solid electrolyte is a big bet for the electric vehicle industry in the coming years, with the promise of practically doubling the autonomy of current vehicles. The electrical energy sector, in which the use of storage systems has become increasingly intense, will also benefit from this new technology, with a reduction in the size and cost of battery banks.
Some advantages of solid electrolyte are increased battery durability, reduced mass and volume and increased safety, since solid electrolyte is not flammable and does not present a risk of explosion, as occurs in liquid batteries.
All of the above advantages are accompanied by reduced manufacturing costs, as the solid electrolyte eliminates the use of separators between electrodes and eliminates the need for sealing coatings (to prevent liquid leakage) and protective casings (to prevent accidental punctures, necessary concern in electric vehicles).
Lithium batteries are commercially available in cells, blocks and banks. The cells are the basic units, which have storage capacities of the order of 1 to 5 Ah, with a nominal output voltage of 3.7 V.
For practical applications these cells need to be organized into blocks or packs, as shown in Figure 5, where they are connected in series. The blocks, in turn, can be connected in parallel to form battery banks.

For application in power systems, smart battery banks with one or more packs of lithium cells associated and integrated with BMS circuits (battery management system) are already found on the market, produced by companies such as Tesvolt, BYD and Dyness – brands commercially available in Brazil.

Flow Batteries
Flow batteries are a slightly different technology from the other electrochemical batteries we know. Instead of the traditional structure shown in Figure 1, in which electrodes are immersed in an electrolyte solution, flow batteries employ electrolytes stored in tanks.
For cells to function, it is necessary to pump electrolytes so that they come into contact with two electrodes separated by a membrane.
This type of battery is viable in large storage systems and is very advantageous due to its high longevity (with more than 10,000 charge and discharge cycles). Its specific energy (Wh/kg) is similar to that of lead-acid batteries. This fact, associated with its construction complexity and low response speed, makes its use unlikely in mobile systems.
Also known as redox (reduction and oxidation) batteries, this family can have different chemical compositions. There are already commercial versions of flow batteries, while they are still the subject of research and have not become as well-known as other technologies already well established on the market.
Liquid Salt Batteries
This type of battery uses electrodes composed of liquid salt. For this to be possible it is necessary to keep the salt heated to high temperatures (around 350 OC) through an internal heating system. This is not very practical for mobile applications, but for stationary power systems the technology is viable and promising.
This type of battery is very advantageous due to its high longevity, and can be stored for many years at room temperature, when its electrodes acquire a solid state. When in operation, keeping the electrodes in a liquid state, they can achieve a large number of charge and discharge cycles.
The best known variations are the sodium sulfur battery (NaS) and the nickel chloride battery (NiCl2). Its specific energy (Wh/kg) is comparable to that of lithium-ion batteries, with the advantage of a long useful life – reaching 4,500 cycles, with a life expectancy of up to 20 years.
Although they are not as well known and are still classified as alternative batteries, there are already commercial applications of this technology in large storage systems.
Good longevity, satisfactory specific energy and reduced cost are characteristics that have attracted attention to this type of battery in BESS applications (battery energy storage systems) on a large scale.
Nickel Batteries
Nickel-cadmium (NiCd) technology has long been widely used in portable electronic equipment. Known for its robustness, it was later replaced by nickel-metal-hydride (NiMH) technology, which has similar characteristics and the main advantage of not using cadmium, a toxic material that makes it difficult to dispose of batteries at the end of their useful life. , in addition to being less subject to the memory effect, which reduces the storage capacity of batteries.
NiMH technology is quite mature and has a long lifetime, high discharge capacity and is economically viable for use in consumer electronics. NiMH batteries are currently found on the market in the format of common batteries and also in other formats for portable and industrial applications. Its use in storage and electric mobility systems is restricted, as its characteristics do not overcome the advantages of lithium-ion batteries.
References
- A review of electrochemical storage technologies for photovoltaic application. Tatiane Silva Costa, Maria de Fatima Rosolem, Marcelo Gradella Villalva (in press)
- Types of Lithium-ion, Battery University, Cadex Electronics
- Batteries in a portable world, Cadex Electronics
- Overview of rechargeable lithium battery systems. Peter Kurzweil, Klaus Brandt. In: Electrochemical power sources: fundamentals, systems and applications, Elsevier, 2019
- Handbook of batteries. David Linden, Thomas B. Reddy. McGraw Hill
- Electric Energy Storage Technology Options: A White Paper Primers on Applications, Costs, and Benefits. Electric Power Research Institute
- Electricity Storage Handbook, Sandia National Laboratories, SAND2013-5131

















4 Responses
Hi Marcelo Villalva
First of all, I’m a BIG fan of your post “Energy Storage: Electric Battery Technologies”
and zinc chrome batteries
Congratulations on the article! One of the only ones with this wealth of details.
Dr Marcelo, many congratulations on the article. It was very useful and quite accessible for most people (I think).
I didn't find the term LiPo (lithium polymers), but I think it fits into one of the categories you mentioned.
I have been using this technology for around 20 years. I was one of the first to use it in Portugal.
The application is in remote-controlled boats (essentially, but also in drones), and I always look for something that gives me the highest c-rate possible, especially for unloading, since these toys are very demanding in this particular requirement. I normally use it, nowadays, with 75C (which I think are real), but I have already found it with 100C.
Another aspect that left me very satisfied was his Portuguese. I don't think I've ever read an article in Portuguese, by a Brazilian, so well written. Many congratulations on that too.
Compliments,
Paulo Capelo