Photovoltaic systems have a long projected lifespan, typically 25 years.
To ensure the longevity of the project we must study and guarantee the durability of all individual components.
One of the most critical components in terms of durability and safety are the photovoltaic module mounting structures.
With varying shapes and applications, we can find them installed in many different ways, in various types of environments and exposed to different weather conditions. Structures can fail in several possible ways.
The main causes of defects are incorrect installation, negligence in sizing calculations (especially due to wind load), inappropriate choices of fixing method, excessive load on the structure and corrosion of the material.

This article aims to show what concerns the designer and installer should have when selecting a structure and the fixing method in relation to the material's durability and corrosion resistance.
Bimetallic corrosion or galvanic corrosion
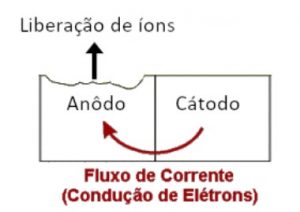
We call galvanic corrosion the electrochemical process in which two metals in contact begin a corrosion process caused by the transfer of electrons in the presence of an electrolyte. All metallic alloys have an electrical characteristic associated with them, referring to the ease with which the metal gives up electrons to the environment in which it finds itself.
The ease of a metal in giving up electrons is called electropositivity, while the tendency to absorb electrons is called electronegativity. According to the nomenclature used in the field of chemistry, the material with greater electronegativity has a greater reduction potential.
It is also said that the more electropositive material has greater reactivity, that is, one material is more reactive than another (has a greater tendency to lose electrons) when it has greater electropositivity.
When a metal or metallic alloy comes into contact with another alloy with different electronegativity, an electrical potential appears between the two materials. With the addition of an electrolyte, which is an aqueous medium capable of conducting ions and electrons, a galvanic cell or battery is formed.
This is the same working principle as a common battery – two distinct metals separated by an electrolyte. The galvanic cell causes a current to flow from the most electropositive material (anode) to the most electronegative material (cathode).
The anodic metal alloy will suffer corrosion, since the process of donating electrons forms ions that can become detached from the metal. For example, an iron alloy acting as an anode undergoes the following chemical reaction:
Fe → Fe2+ + 2e
This reaction removes the iron from the metal alloy and makes it available in the form of iron ions to the electrolyte. The iron ion combines with oxygen in the air and causes rust (iron oxide II and III mainly). Meanwhile, at the cathode, the metal receives the electrons given up by the anode.
Galvanic corrosion occurs significantly when there is a difference in electronegativity of the alloys in contact greater than 0.5V. The table below shows the electronegativity values relative to gold of the most common metallic alloys.
Table 1 – Electronegativity values of common alloys in relation to gold

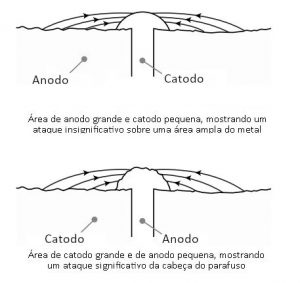


If a screw is in contact with another material that makes it an anode, it corrodes quickly, increasing the risk of breakage and loosening of the tightness. In short, for galvanic corrosion to occur, it is necessary that:
- There is an electrolyte connecting the two metals: the electrolyte in the case of structures exposed to the weather is water, air humidity and sea air;
- Electrical contact between the two metals: if there is an insulator between the two metals, there will be no path for the galvanic current to flow, therefore galvanic corrosion does not occur;
- A difference in electropositivity or electronegativity greater than 0.5 V.
Galvanic corrosion protection
There are measures that can be adopted to prevent galvanic corrosion of metal structures.
The first measure, which is not even possible, is to use metals that are compatible with each other, that is, those that have an electronegativity difference of less than 0.5 V. It is not always possible to previously analyze each metal combination used in the structure to be assembled in field on the client.
A second measure may be the selection of materials such that the anode has a much larger area than the cathode. When this occurs, corrosion is reduced and may become insignificant, depending on the relationship between the areas and the absence of electrolyte (in regions with a dry climate, with little humidity in the air).
If it is not possible to choose compatible materials, one solution is to ensure that the metal parts of a structure have some treatment that prevents galvanic corrosion. This treatment can be adding a layer of zinc to the steel, anodizing the aluminum or carrying out special surface treatments.
The zinc layer on galvanized steel or galvanized steel protects the steel from contact with oxygen and other external sources of corrosion. Zinc also acts as a sacrificial anode if this barrier is broken, giving electrons to the steel and preventing it from disintegrating.

The installation location is a determining factor in the durability of galvanized structures. The installation location directly influences the thickness of the zinc layer to be applied.
The zinc layer corrodes over time and can expose the steel to corrosion if the correct thickness is not observed. The corrosion rate of the zinc layer directly depends on the environment in which it is found and the metal it protects, as shown in the table below.
Table 2 – Estimated corrosion of the zinc protective layer for each type of metal in contact and installation environment, in micrometers per year

In the figure below, we see an example of corrosion accentuated by sea spray in a coastal location. The photograph specifically shows a case of bimetallic corrosion due to the contact of grounding terminals with the frame of the photovoltaic module.
Typically, these connectors are made of copper, bronze (tin and copper alloy) or brass (copper and zinc alloy), which have greater electronegativity than aluminum. Being more electropositive, aluminum tends to disintegrate.

In some cases, it is possible to carry out other types of surface treatments or adopt strategies to isolate incompatible metal parts. If the metals are isolated from each other or isolated from the electrolyte, there is no longer any way for the anode to give up electrons to the cathode.
This insulation can be chemical (with the surface treatment of the parts) or physical (where the metallic parts are separated by an insulator). The physical solution is not always viable, as it may represent an increase in the cost of the work and the need for more frequent maintenance in order to check the quality of the insulation.

Aluminum alloys can be protected by growing an additional layer of oxide on their surface. The oxide that is formed acts as an electrical insulator, thus reducing current flow and bimetallic corrosion when in contact with another alloy.
Aluminum treated in this way is called anodized aluminum. Because of its good durability and compatibility, anodized aluminum is the most common material in the construction of photovoltaic module frames and fixing structures.
Despite its advantages, aluminum is expensive and can be successfully replaced in some cases by steel. subjected to anti-corrosive surface treatments such as galvanizing, phosphating and electrophoretic painting.
The protective layer created by surface treatments must prevent material contact due to the presence of electrolyte, typically formed by condensate, dust, dew, rain, wet residues in contact with metals, soil moisture and others.
With the isolation between metals, the galvanic cell is not formed, thus interrupting the exchange of electrons, generation of ions and galvanic corrosion. An important point in the treatments on structures for photovoltaic systems is UV (ultraviolet) protection compatible with the other components.
The structures are exposed and therefore subject to an important risk factor in defining the corrosion protection used. The most common surface treatments found in the industry and which can also be applied in the photovoltaic segment, in addition to traditional galvanization, are phosphating and cathodic electrophoresis.
Phosphating is a surface treatment widely used in the automobile industry, which offers good anti-corrosion protection and good adhesion to paintwork. Phosphating is normally used as preparation for a final surface treatment, as is the case with cathodic electrophoresis (KTL), which adds a film of organic pigments and gives metal parts extraordinary protection against corrosion.

Corrosion in soil structures and grounding systems
All solar systems require grounding. In systems implemented in the ground, we have the metal of the structure buried and the grounding mesh, which are typically made of different metals and can corrode between them.
The grounding grid is typically made of bare copper buried directly into the ground and the buried metal structure is typically aluminum or steel. Both metals in the structure form galvanic batteries with copper, however the relationship between the areas of the metals is such that the corrosion caused by the association is small.
For additional fixation and durability purposes, alloy steels are commonly attached to buried concrete footings. Concrete forms a kind of film on the steel that reduces the effects of galvanic corrosion on the material.
This does not eliminate the concern about galvanic corrosion, as connections to the grounding system must still be made with compatible metals. Materials can also suffer other corrosion processes related to the acidity and composition of the soil.
Corrosion in photovoltaic systems
The designer and installer must be careful when selecting the structure for a photovoltaic system. Corrosion of structures in these systems occurs mainly due to:
- Incompatibility between the metals of the structure, module and roof;
- Choosing incompatible screws. For example, zinc-plated steel screw in stainless steel structure;
- Damage to the anodizing layer of aluminum or galvanized steel;
- Metals unsuitable for locations with significant salt spray.

Conclusion
The appropriate choice of materials for the construction of fixing structures is an important factor in determining the useful life of photovoltaic systems. Screws, grounding clips and structural parts must be chosen according to the electronegativity of each material.
The installation environment must also be taken into account. Oceanic regions, with the strong presence of salinity, can present accelerated corrosion of materials. In all cases, it is important to check the type of treatment used by structure manufacturers, such as the KTL process (cathodic electrophoresis), the Geomet process and others.